Report by
Recall notice
ML Natural Lactoferrin as Apolactoferrin recalled due to Undeclared Allergen
9 months ago •source fda.gov
United States
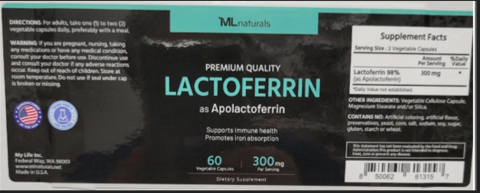
My Life Inc. of Federal Way, WA, is recalling approximately 65 bottles of ML Naturals Premium Quality Lactoferrin as Apolactoferrin 300mg. capsules dietary supplement, lot FL2407511L19, expiration date 10/2027, because it may contain undeclared milk. People who have an allergy or severe sensitivity to milk run the risk of a serious or life-threatening allergic reaction if they consume this product. The product was sold directly to internet consumers from the Amazon website, and consumers also received the product through the Amazon VINE Program with the ASIN #BODNLMBHGG and SKU #FC-6UFC-K026.The recalled Lactoferrin as Apolactoferrin dietary supplement has the lot FL2407511L19, expiration date 10/2027, UPC 850062 613157. The product is packaged in a black plastic bottle and a black lid, and each bottle contains 60 vegetable capsules, 300mg per capsule.
The recall was initiated after it was discovered during the FDA inspection that a product containing milk was distributed in packaging that did not reveal the presence of milk. This recall is being made with the knowledge of the U.S. Food and Drug Administration.
Consumers who have purchased the affected product and have a milk allergy or sensitivity are urged not to consume the product and return it for a full refund or dispose of it safely if returning the product is not possible.
Source: www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/my-life-inc-issues-allergy-alert-undeclared-milk-ml-natural-premium-quality-lactoferrin
Comments
Comment